Introduction
Waldenström's macroglobulinemia (WM) is an indolent lymphoma with medullary infiltration by a lymphoplasmacytic clone associated with an immunoglobulin M paraprotein. Some patients diagnosed with WM present a concomitant inflammatory syndrome: increased C-reactive protein (CRP) levels, fever, recurrent night sweats, anorexia. These patients were usually referred as inflammatory WM despite no definition has been established. Here, we describe clinical and biological characteristics of inflammatory WM patients, response to treatment, survival and discuss pathogenesis hypotheses.
Methods
In this descriptive retrospective study, we included WM patients followed at Saint-Louis hospital between 2007 and 2019 with WM diagnosis fulfilling the World Health Organization's criteria, with medullary infiltration proven by bone marrow biopsy or aspiration examination and monoclonal M paraprotein, associated with a biological inflammatory syndrome with or without clinical inflammatory signs. Inflammatory WM diagnosis was retained in the absence of differential diagnoses for the inflammatory syndrome such as cancer, infection, autoimmune or inflammatory diseases, using complete blood workup, PET and body scanner.
Results
Two-hundred and forty-two patients were included: 67 of inflammatory phenotype (28%), 166 non-inflammatory (68%) and 9 with inflammatory syndrome of unknown origin (4%). Describing inflammatory WM, diagnoses were made between 1985 and 2018, 50% after 2007. At treatment initiation, median age was 69 years (range 45-94), monoclonal IgM median value was 24.9 g/L (4.8-77.3) (Table 1). Median CRP was 40.5mg/L (IQR 23.8-64.8). CRP was correlated with fibrinogen (r =0.53, 95% confidence interval (CI) 0.18-0.76, p=0.006) and inversely correlated with albuminemia (r=-0.65, CI -0.82- -0.39, p < 0.001). Mean medullary infiltration was 50%. Three of 35 patients with avalaible karyotype had 6q deletion. Only 12 patients had medullary molecular analysis: 9 of them harboured MyD88 L265P mutation, 2 p53 mutations and none CXCR4 WHIM mutation. Five cryoglobulin tests were positive, Coombs test positive for 3 patients and anti-MAG antibodies positive for 2.
Indications for front-line therapy were cytopenias in 44 patients (88%), inflammatory syndrome for 20 (40%), systemic symptoms in 15 (30%), tumoral syndrome in 10 (20%) and hyperviscosity syndrome in 3 (6%). Sixty-two patients were treated, at front-line 60% received rituximab-based therapy and 39% monotherapy. Overall response rate was 84%. Inflammatory and hematological response were statistically correlated (OR 13.8, CI 2.07-74.6, p = 0.005).
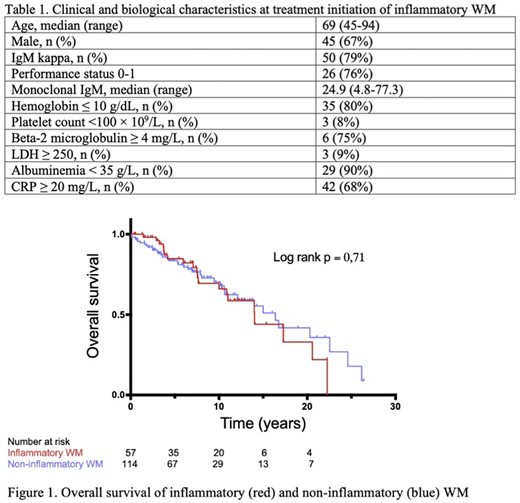
Median follow-up was 12.6 years (IQR 7.0-22.8). Median progression-free survival (PFS) after front-line therapy was 32 months and estimated PFS was 68% (CI 55-78) at 10 months, 59% (CI 46-70) at 20 months and 30% (CI 19-42) at 5 years. Overall survival (OS) for inflammatory and non-inflammatory WM patients were not significantly different (median OS 14.0 and 16.4 years, respectively, c2 0,14, p=0,71, Figure 1). At 5 years, estimated OS was 85% (CI 71-93) inflammatory patients and 84% (CI 75-90) for non-inflammatory, at 10 years 66% (CI 48-79) and 70% (CI 58-79). Three patients were secondarily diagnosed with diffuse large B-cell lymphoma (DLBCL).
Conclusions
We reported 67 WM patients with inflammatory phenotypes, representing a third of WM patients in our cohort. Compared with literature, their clinical and biological characteristics do not differ from non-inflammatory WM patients, as well as response to treatment and PFS. There is no excess of transformation into DLBCL. Our inflammatory and non-inflammatory cohorts show no difference in terms of OS. Inflammatory response is strongly correlated with hematological response. Non-decrease of CRP level during treatment and increase of CRP during follow-up in inflammatory WM patients should be a warning sign for refractory disease or relapse.
Further studies are needed to better delineate this sub-group of patients and explore its pathogenesis. Studying medullary cytokinic environment, WM cells, tumoral microenvironment and M paraprotein contributions could elucidate the underlying pathophysiological mechanisms involved.
Thieblemont:Roche, Hospita: Research Funding; Roche, Amgen, Kyte Gilead, Celgene, Abbvie, Novartis, Cellectis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Cellectis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal